Gas detection is a crucial aspect of safety in various industrial and environmental settings. It involves measuring the concentration of potentially hazardous gases to prevent accidents, ensure compliance with safety standards, and protect human health. Three common units used in gas detection are %LEL, %LFL, % vol and ppm. Understanding the differences between these units and knowing how to convert between them is essential for the gas detection industry.
Understanding the units
%LEL (percent of lower explosive limit)
%LEL stands for percent of the Lower Explosive Limit (LEL). The Lower Explosive Limit is the lowest concentration of a gas or vapor that can ignite in air. LEL value of a gas can vary depending on a region. The %LEL indicates how close the gas concentration is to this flammable threshold. For instance, a reading of 50 %LEL means the gas concentration is at half the minimum concentration required for combustion.

%LFL (percent of lower flammable limit)
%LFL refers to the same minimum concentration of gas or vapor in air that can ignite. The difference lies in the context: LEL is mostly used in explosion prevention, while LFL is more relevant to fire hazards.
In practical terms, LEL and LFL are the same values, and both are typically expressed as a percentage by volume in air (e.g., 4.4% of methane) or in parts per million (44 000 ppm of methane).
% vol (percent by volume)
Percent by volume (% vol) stands for percent by volume and represents the concentration of a gas in a mixture. It is expressed as a percentage of the total volume. For example, if a gas concentration is 2% vol, it means that the gas makes up 2% of the total volume of the air or gas mixture.

ppm (parts per million)
Parts per million (ppm) stands for parts per million and is a unit of measurement used to express the concentration of a gas in a mixture. One ppm represents one part of the gas per one million parts of the total volume. This unit is particularly useful for measuring very low concentrations of gases.
Relationship and сonversions
The relationship between %LEL, %LFL, % vol and ppm are crucial for gas detection and safety management. However, converting between these units requires specific information, for example, Lower Explosive Limit (LEL) and Lower Flammable Limit (LFL) values.
Conversion from %LEL / %LFL to ppm
To convert %LEL / %LFL to ppm, you need to know the Lower Explosive Limit (LEL) / Lower Flammable Limit (LFL) value of the gas in ppm. The formula is:

For example, if the Lower Explosive Limit (LEL) / Lower Flammable Limit (LFL) of hydrogen is 40 000 ppm, then 10 %LEL / %LFL of hydrogen is: 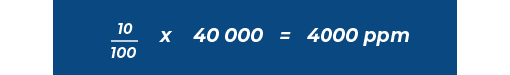
Conversion from % vol to %LEL / %LFL
To convert % vol to %LEL / %LFL, you need to know the Lower Explosive Limit (LEL) / Lower Flammable Limit (LFL) value in % vol:

For example, if the Lower Explosive Limit (LEL) / Lower Flammable Limit (LFL) of hydrogen is 4% vol, then 0.4% vol of hydrogen is:
Various gases Lower Explosive Limit (LEL) / Lower Flammable Limit (LFL) values in % vol can be found in the following standards:
- ISO 10156:2017 Gas cylinders - Gases and gas mixtures - Determination of fire potential and oxidizing ability for the selection of cylinder valve outlets (ISO 10156:2017)
- ISO/IEC 80079-20-1 Explosive atmospheres - Part 20-1: Material characteristics for gas and vapour classification - Test methods and data
Examples of LEL / LFL values in % vol for some gases according to ISO/IEC 80079-20-1:
| Gas | LEL / LFL (in % vol) |
|---|---|
|
Acetone
|
2.5 |
| Acetylene | 2.3 |
| Ammonia | 15.0 |
| Butane | 1.4 |
| Ethanol | 3.1 |
| Hydrogen | 4.0 |
| Isobutylene | 1.6 |
| Methane | 4.4 |
| Propane | 1.7 |
| Propylene | 2.0 |
| Toluene | 1.0 |
| Xylene | 0.9 |
Conversion from % vol to ppm
The conversion from % vol to ppm is straightforward:

For example, hydrogen (H2) concentration of 4% vol would be equivalent to:

Each unit provides a different perspective on gas concentration, from high-level percentages by volume to lower parts per million.
By mastering these conversions, industry professionals can be sure about the values they operate with, contributing to safer industrial operations and a more controlled environment.